Development of SLC transporter drugs
We select promising transporters from among SLC transporters as drug targets and analyze their functions and structures, which leads to drug discovery.
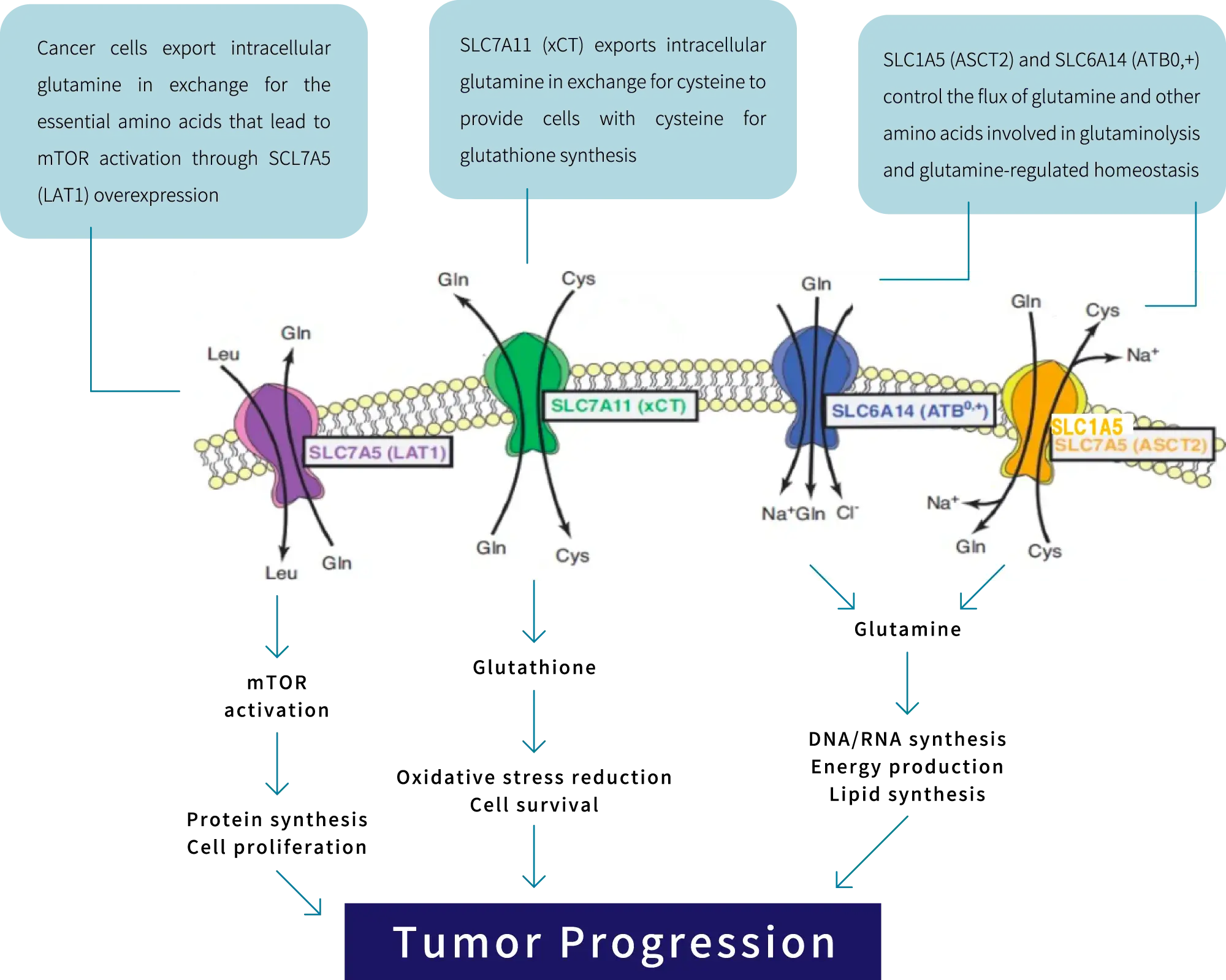
SLC (Solute Carrier) transporters are proteins that exist in the cell membrane and efficiently transport organic substances essential for the maintenance of living organisms, such as glucose and amino acids, into the cell. To date, more than 400 SLC transporter genes have been identified and are being investigated as therapeutic targets for diseases such as cancer, autoimmune diseases, metabolic diseases, and neurodegenerative disorders.
Examples of SLC transporters
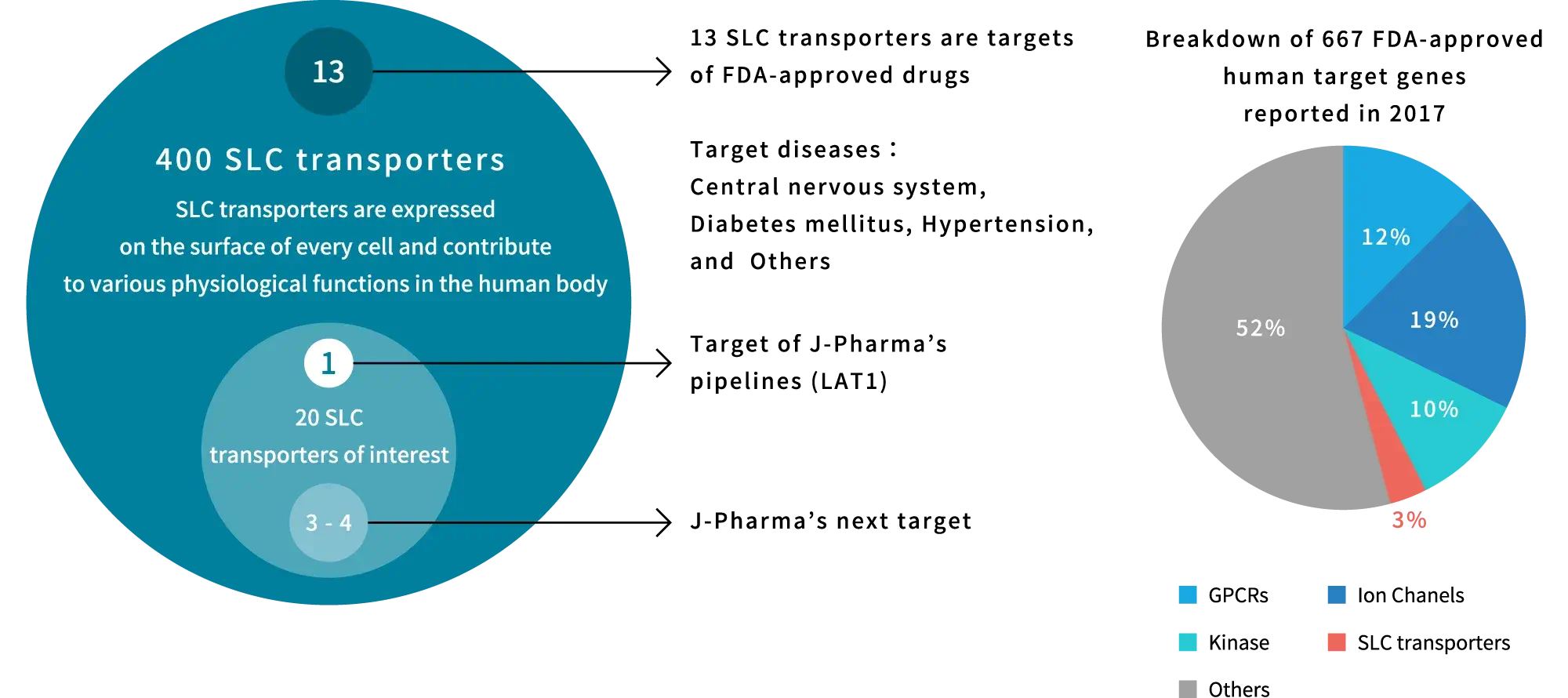
Although SLC transporters have been considered potential therapeutic targets for a variety of diseases, the development of drugs targeting SLC transporters has been slow because SLC transporters possess multiple, diverse functions and complex molecular configurations and thus remained obscure until recent years. As a result, only 13 of the more than 400 identified SLC transporters have been targeted by U.S. FDA-approved drugs, and the percentage of target genes in U.S. FDA-approved drugs is very small, less than 5% (based on data as of 2017). On the other hand, recent advances in structural analysis, computational biology, and other areas have led to rapid progress in elucidating the functions of hidden SLC transporters, making drug development possible.
While the development of SLC transporter-targeted therapeutics has begun to accelerate in recent years, since our founder started research on SLC transporters in 1993, we have continued to develop and expand our research on SLC transporter-targeted therapeutics and demonstrated clinical trial results that make us a leader in SLC transporter drug discovery. Furthermore, we have developed and built upon this drug discovery technology as a platform.
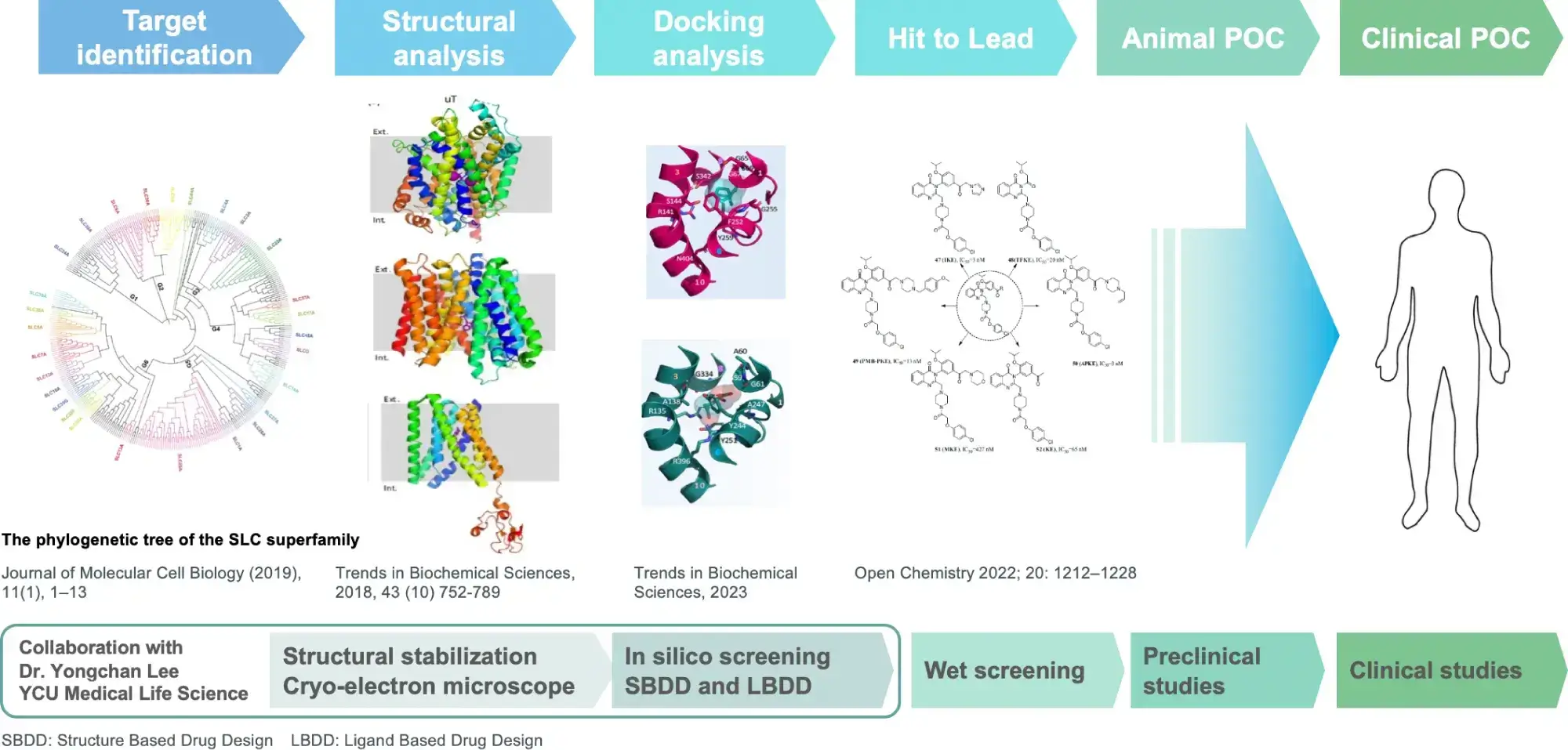
By evaluating 61 amino acid transporters involved in physiological and pathological processes, our founder made the molecular identification of LAT1 (cancer-specific essential amino acid transporter) in 1998 and URAT1 (uric acid transporter that reveals the cause of hyperuricemia) in 2002. Among these, our research initially focused on LAT1, leading to the discovery of nanvuranlat in 2003, a compound selectively inhibiting LAT1. We initiated a Phase 1 study of nanvuranlat in 2015, and in 2022, we successfully completed a Phase 2 study (as a second line or later therapy for biliary tract cancer), demonstrating its efficacy and safety.
During the research and development of nanvuranlat, in 2019, we achieved the elucidation of the complex structure of LAT1 and CD98 by cryo-electron microscopy in collaboration with Yokohama City University. Since the molecular assembly of LAT1 and the mechanism of amino acid transport are still unclear, this structural elucidation has greatly advanced the possibilities for future drug discovery. LAT1 features a canonical LeuT-fold while exhibiting an unusual loop structure on transmembrane helix 6, creating an extended cavity to accommodate bulky hydrophobic amino acids and drugs. CD98hc engages with LAT1 through multiple interactions, not only in the extracellular and transmembrane domains but also in the interdomain linker.
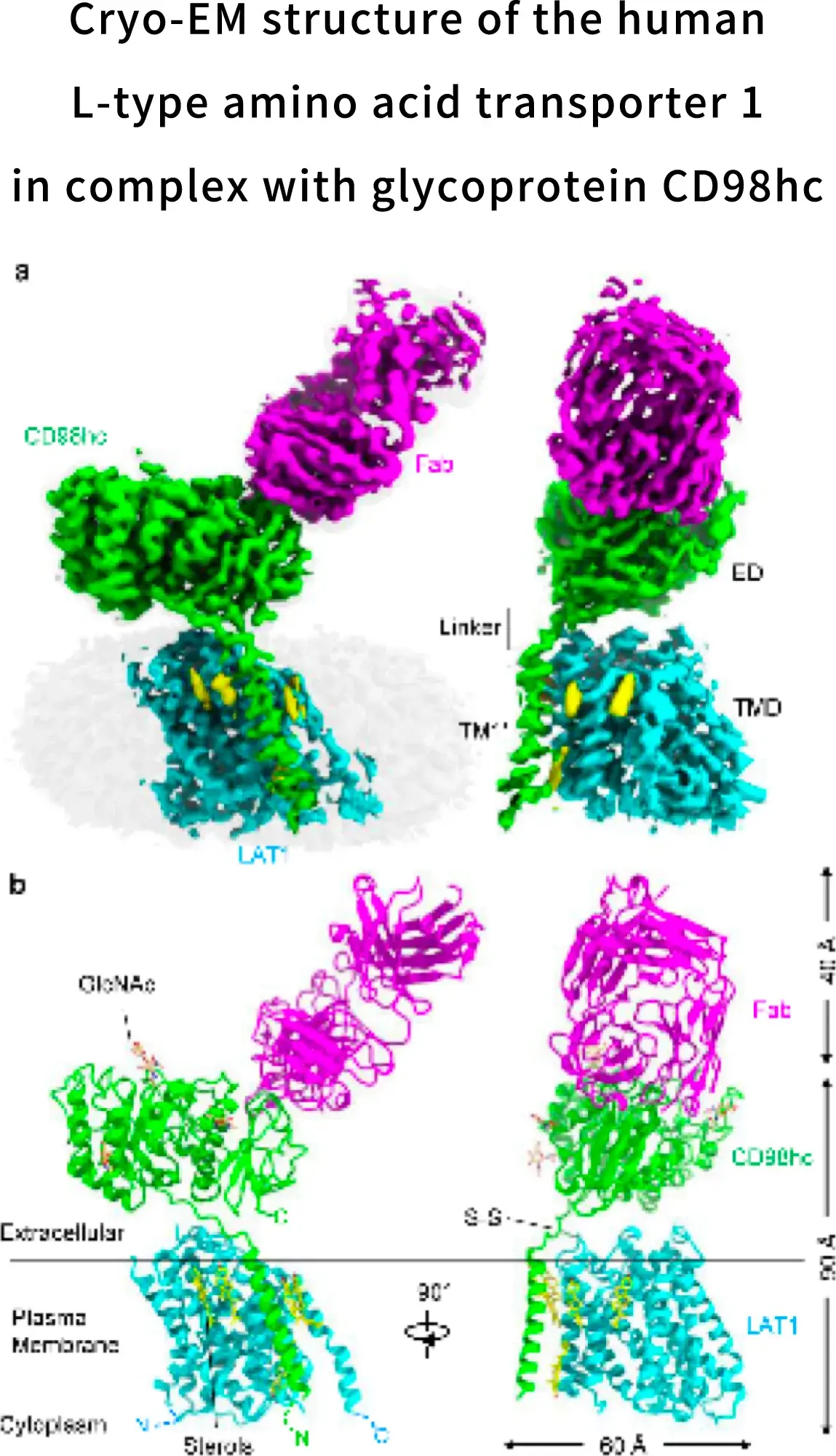
Lee, Y., Nat Struct Mol Biol 26, 510–517 (2019)
Alongside the two LAT1 inhibitors, nanvuranlat and JPH034, which are currently under development, we have identified a candidate compound for future LAT1 inhibitors. Additionally, we have initiated drug discovery research on the next SLC transporters, which represent potential anti-cancer drug targets.
LAT1 inhibitors
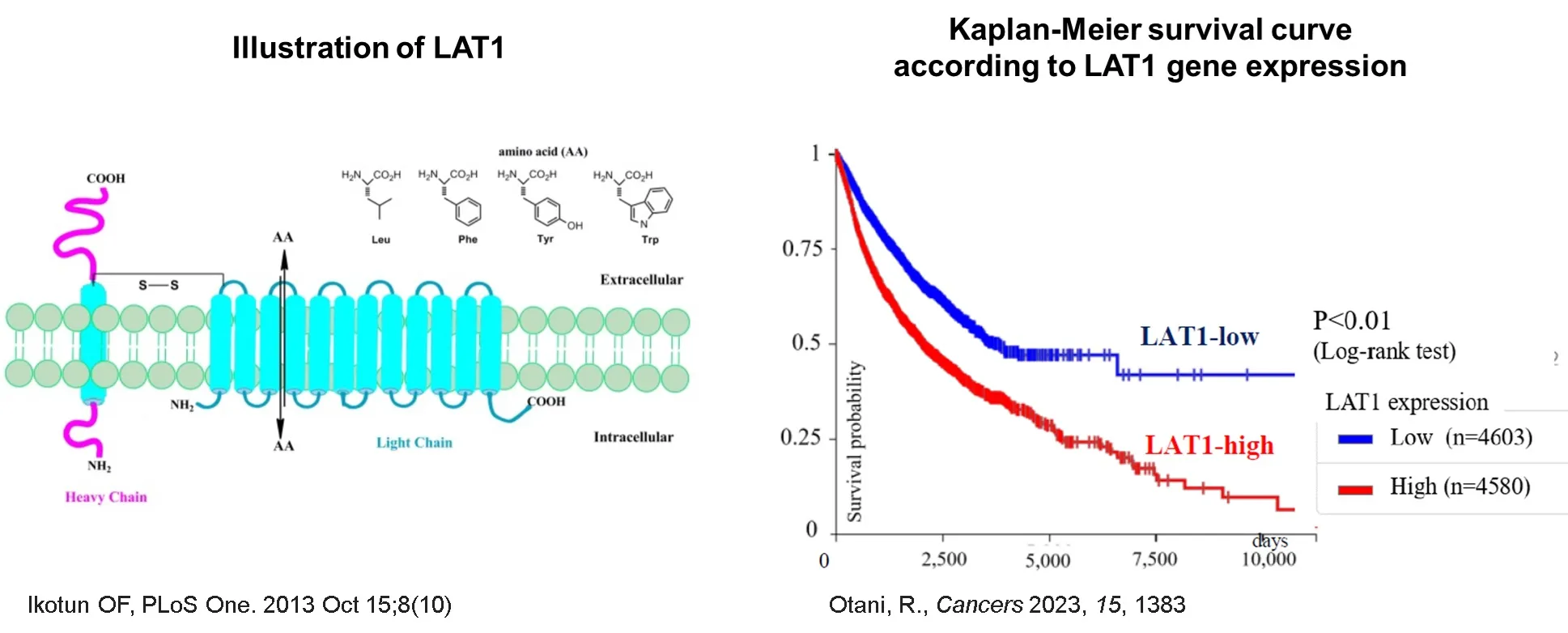
One of the SLC transporters, LAT1 (L-type amino acid transporter 1), discovered by our founder in 1998, is a transporter that transports large neutral amino acids and has been confirmed to contribute to cancer cell growth and immune cell activation in autoimmune diseases.
Diseases that have been identified as being associated with LAT1
| Cancers | Autoimmune diseases and others |
|---|---|
| Biliary tract cancer, Colon cancer, Kidney cancer, Pancreas cancer, Brain tumor, Breast cancer, Thyroid cancer | Multiple sclerosis, Systemic lupus erythematosus, Chronic neuropathic pain, Retinal angiogenesis, Atopic dermatitis, Inflammation |
Cancer treatments and LAT1
LAT1 is overexpressed in a variety of solid tumors, and it is known that the higher the expression of LAT1, the shorter the survival time. When cells become cancerous or proliferate rapidly, LAT1 expression on the plasma membrane increases, leading to enhanced amino acid uptake and rapid cell growth. LAT1 expression is closely correlated with lymphatic metastasis, cell proliferation, angiogenesis, and shortened survival.
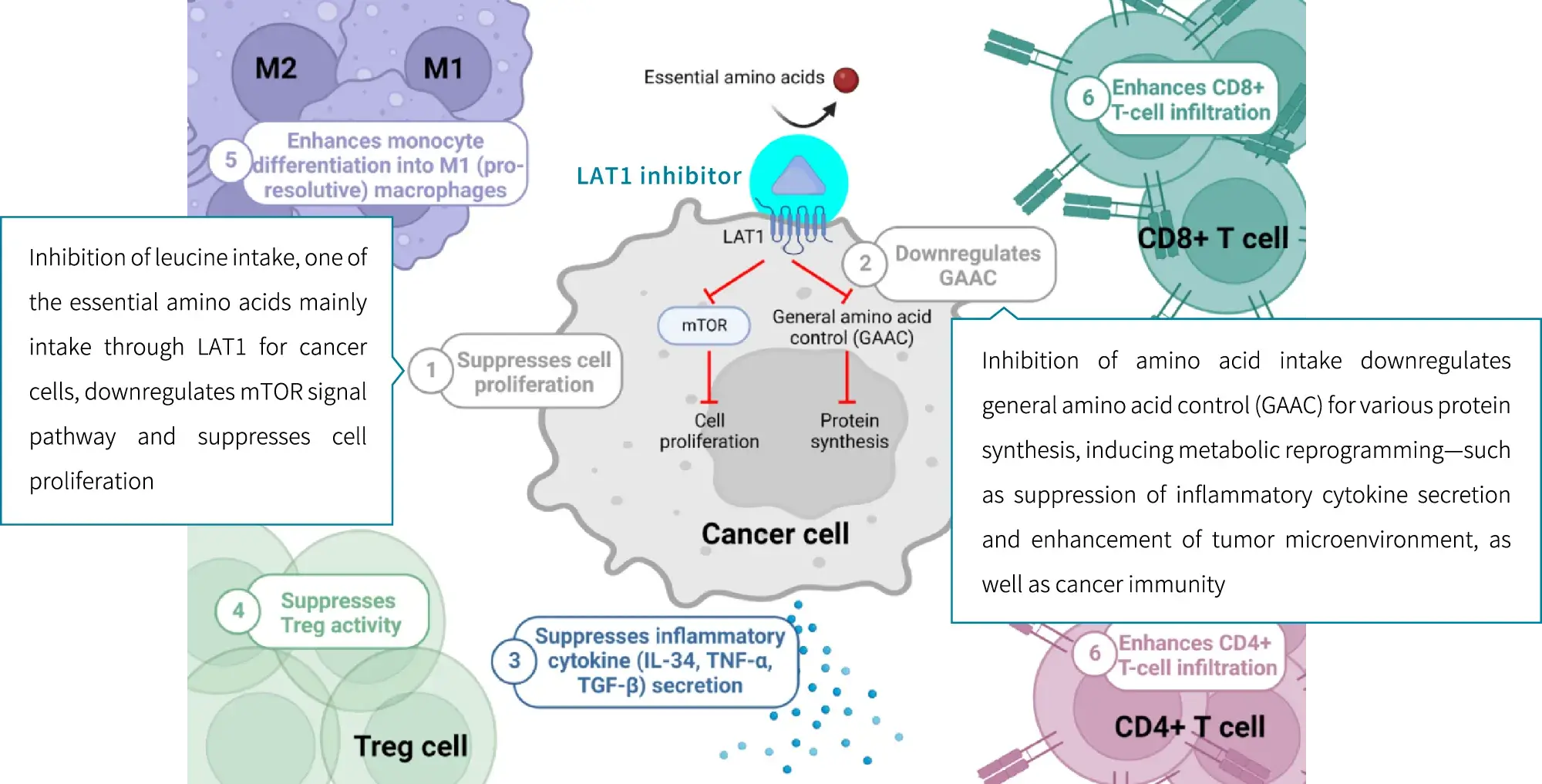
LAT1 inhibitors are considered to be able to inhibit cancer cell growth through multiple pathways by blocking LAT1-mediated uptake of amino acids by cancer cells.
Autoimmune diseases and LAT1
When immune cells are activated, LAT1 gathers on their surface, uptakes large amounts of amino acids, and releases inflammatory cytokines to trigger inflammation. LAT1 is reported to be expressed only when lymphocytes, macrophages, dendritic cells, B cells, etc. are activated. Inhibiting the function of LAT1 with LAT1 inhibitors may lead to the treatment of various autoimmune diseases by suppressing inflammation.
Presentations at Conferences and Publications
J-Pharma’s Presentations at Conferences, Publication and Key Related Work
Nanvuranlat, an L-type amino acid transporter (LAT1) inhibitor for patients with pretreated advanced refractory biliary tract cancer (BTC): Primary endpoint results of a randomized, double-blind, placebo-controlled phase 2 study.
<URL:https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.4_suppl.494?af=R>
2023 ASCO Annual meeting, Clinical Science Symposium Subgroup analysis of a double-blind, placebo-controlled Ph. 2 study of nanvuranlat in treatment of pre-treated, advanced, refractory biliary tract cancer (BTC): Patients with high LAT1 expression and response to nanvuranlat.
<URL:https://ascopubs.org/doi/10.1200/JCO.2023.41.16_suppl.4011>
Kaira et al., Clinical significance of L-type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therapy in biliary tract cancer. BMC Cancer 2013, 13, 482
<URL:https: //bmccancer.biomedcentral.com/articles/10.1186/1471-2407-13-482>
Supak et al., Inhibition of l-type amino acid transporter 1 activity as a new therapeutic target for cholangiocarcinoma treatment, Tumor Biology 2017 Mar;39(3) 1-14
<URL:https://pubmed.ncbi.nlm.nih.gov/28347255/>
Nobuyuki et al., High expression of L-type amino acid transporter 1 as a prognostic marker in bile duct adenocarcinomas, Cancer Medicine 2014; 3(5): 1246–1255
<URL:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4302674/>
Palanivel et al., Oncogenic KRAS mutations enhance amino acid uptake by colorectal cancer cells via the hippo signaling effector YAP1, Mol. Oncol. 2021; 15. 2782–2800
<URL:https://pubmed.ncbi.nlm.nih.gov/34003553/>
Arafath et al., The amino acid transporter SLC7A5 is required for efficient growth of KRAS-mutant colorectal cancer, Nature Genetics 2021 Jan; 53 (16) 16–26
<URL:https://pubmed.ncbi.nlm.nih.gov/33414552/>
Ainara et al., HIF2α acts as an mTORC1 activator through the amino acid carrier SLC7A5, Molecular Cell 2012 Dec 14;48(5), 681–691
<URL:https://pubmed.ncbi.nlm.nih.gov/23103253/>
Kosuke et al., Characterization of the expression of LAT1 as a prognostic indicator and a therapeutic target in renal cell carcinoma, Sci Rep. 2019 Nov 20;9(1): 16776
<URL:https://pubmed.ncbi.nlm.nih.gov/31748583/>
Altan et al., Relationship between LAT1 expression and resistance to chemotherapy in pancreatic ductal adenocarcinoma. Cancer Chemotherapy and Pharmacology 2018; 81. 141–153.
<URL:https://pubmed.ncbi.nlm.nih.gov/29149426/>