Development status
We are focusing on L-type amino acid transporter 1 (LAT1), an essential amino acid transporter discovered by J-Pharma’s founder, and developing several compounds (LAT1 inhibitors) for different indications.

Nanvuranlat
Nanvuranlat (JPH203) is a competitive LAT1 inhibitor that was discovered by Dr. Hitoshi Endou, M.D., Ph.D., the founder of J-Pharma, and his colleagues as a novel small molecule targeting LAT1. J-Pharma has been conducting research and development. With its unique biodistribution across organs such as the bile and kidneys through specific transporters, nanvuranlat has the potential to prevent cancer proliferation by inhibiting the activity of LAT1. Nanvuranlat has the potential to be a novel treatment for patients with specific solid tumors who are not covered by marketed drugs.
Nanvuranlat completed a Phase 2 study in 2022 for its lead program of BTC monotherapy. The results were presented orally at the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium 2023 and the 2023 ASCO Annual Meeting, where the top few percent of abstract submissions have opportunities to present orally and are highly acclaimed by KOLs around the world. Based on the study results, we are preparing for the next phase of development in each major country. In the U.S., we have received orphan drug designation and are in pre-IND meetings with the U.S. FDA for a global Phase 3 study.
Phase 2 study (monotherapy for second-line and later BTC)
[Study Design]
A randomized, double-blind, placebo-controlled Phase 2 study of nanvuranlat in patients with previously treated advanced or refractory biliary tract cancer. Fourteen facilities in Japan participated. Consent was obtained from 211 patients, of whom 104 were randomized, with 69 receiving nanvuranlat and 35 receiving placebo, after being stratified based on the polymorphism of the drug-metabolizing enzyme (NAT2). The study enrolled cases of four different subtypes of biliary tract cancer (intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, gallbladder cancer, and ampulla of vater carcinoma), and 83% of patients had been treated with two or more therapies prior to enrollment. The primary endpoint was Progression-Free Survival (PFS) as assessed by a blinded independent central review (BICR) based on the new guidelines for determining therapeutic response in solid tumors (RECIST 1.1). Key secondary endpoints included overall survival (OS) and disease control rate (DCR: CR+PR+SD).
[Summary of the results]
- Nanvuranlat demonstrated a statistically significant PFS benefit in advanced or refractory BTC patients who had received prior therapy over placebo with HR 0.56 (0.34 – 0.90, 95% CI, p = 0.016)* and met primary end points
- LAT1 high-expressing patients enhanced the PFS benefit over placebo with HR 0.44 (0.23 – 0.85, 95% CI, p = 0.01)*, even though the LAT1 high population has been documented as having a poor prognosis
- Nanvuranlat works very effectively on extrahepatic cholangiocarcinoma (EHC) and gallbladder cancer (GBC), which currently have no second-line treatments available in the US/EU, with a HR 0.22 (0.10 – 0.49, 95% CI, p = 0.001)*
- Nanvuranlat demonstrated a cleaner safety profile compared to marketed BTC drugs (all samples: Nanvuranlat 41.4%, Placebo 57.1%, ≥Grade 3 samples: Nanvuranlat 30.0%, Placebo 22.9%) without events leading to discontinuation, dose reduction or death
- Hazard ratio is a statistical term that is an objective way of comparing relative risks. When the hazard ratio is 1 for Drug A and Drug B compared in a clinical trial, there is no difference between the two treatments; when the hazard ratio is less than 1, Drug A is determined to be more effective, and the smaller the value, the more effective it is. Confidence intervals and p-values are numbers that indicate the reliability of the calculated statistical data.
Conference presentation
Gastrointestinal Cancers Symposium: ASCO GI 2023
Nanvuranlat, an L-type amino acid transporter (LAT1) inhibitor for patients with pretreated advanced refractory biliary tract cancer (BTC): Primary endpoint results of a randomized, double-blind, placebo-controlled phase 2 study.
<URL: https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.4_suppl.494?af=R>
2023 ASCO Annual Meeting, Clinical Science Symposium
Subgroup analysis of a double-blind, placebo-controlled Ph. 2 study of nanvuranlat in the treatment of pre-treated, advanced, refractory biliary tract cancer (BTC): Patients with high LAT1 expression and response to nanvuranlat.
<URL: https://ascopubs.org/doi/10.1200/JCO.2023.41.16_suppl.4011>
Biliary tract cancer (BTC) and nanvuranlat
More than 200,000 people worldwide are diagnosed with BTC each year, but it is often asymptomatic in its early stages, and most new patients are diagnosed at an advanced stage. Advanced-stage biliary tract cancer has a poor prognosis due to limited treatment options, and the 5-year survival rate is extremely low, at 5-15%.
BTC is comprised of four heterogenous cancers: Intrahepatic (IHC), Extrahepatic (EHC), Gallbladder (GBC) and Ampulla of Vater (AVC). Chemotherapy and PD-L1 antibodies are the only approved treatment options in the first-line across various BTC subtypes today. Molecularly targeted drugs are approved worldwide as second-line treatments, however, the genetic mutations targeted by those targeted drugs occur almost exclusively in IHC, and even in IHC, molecularly-targeted drugs are only available for less than 15% of patients. In addition, an analysis of real-world data using U.S. insurance claims data shows that the majority of the patients do not receive active treatment with chemotherapeutic agents in first-line therapy and receive palliative care. Thus, there is a need for new drugs that are well-tolerated and safe enough to be used in the treatment of BTC in a wide range of patients.
Because it selectively inhibits only LAT1, which is specifically expressed in cancer cells, nanvuranlat is safe and well tolerated, potentially providing a new treatment option for BTC and addressing patients’ needs.
Furthermore, nanvuranlat has been shown to improve cancer immunity and enhance the long-term function of immune cells against cancer cells, and non-clinical data confirmed that nanvuranlat enhances efficacy as a combination therapy of existing immune checkpoint inhibitors (such as anti-PD-L1 antibodies). By promoting the development of combination therapy with anti-PD-L1 antibody drugs, nanvuranlat potentially contributes to the treatment of more BTC patients and patients with other solid tumors.
Colorectal cancer, renal cell carcinoma, and nanvuranlat
Nanvuranlat is being developed for the potential expansion of indications to colorectal cancer and renal cell carcinoma based on non-clinical and clinical data. For colorectal cancer, clinical data have been published showing that high expression of LAT1 is strongly associated with a poor prognosis in patients with certain mutated colorectal cancers. Non-clinical data have also been published showing that nanvuranlat has a significant growth suppressive effect in the same mutated colorectal cancer cell lines. In addition, Phase 1 studies, conducted for five types of solid tumors, showed some positive results in colorectal cancer, and further development is expected. For renal cell carcinoma, similar to colorectal cancer, non-clinical data have been published showing a clear association between high LAT1 expression and poor prognosis, as well as demonstrating that nanvuranlat inhibits the growth of renal cell carcinoma cell lines in a dose-dependent manner. Additionally, nanvuranlat has drug characteristics that result in high distribution within kidney tissues, where renal cell carcinoma is predominant.
JPH034
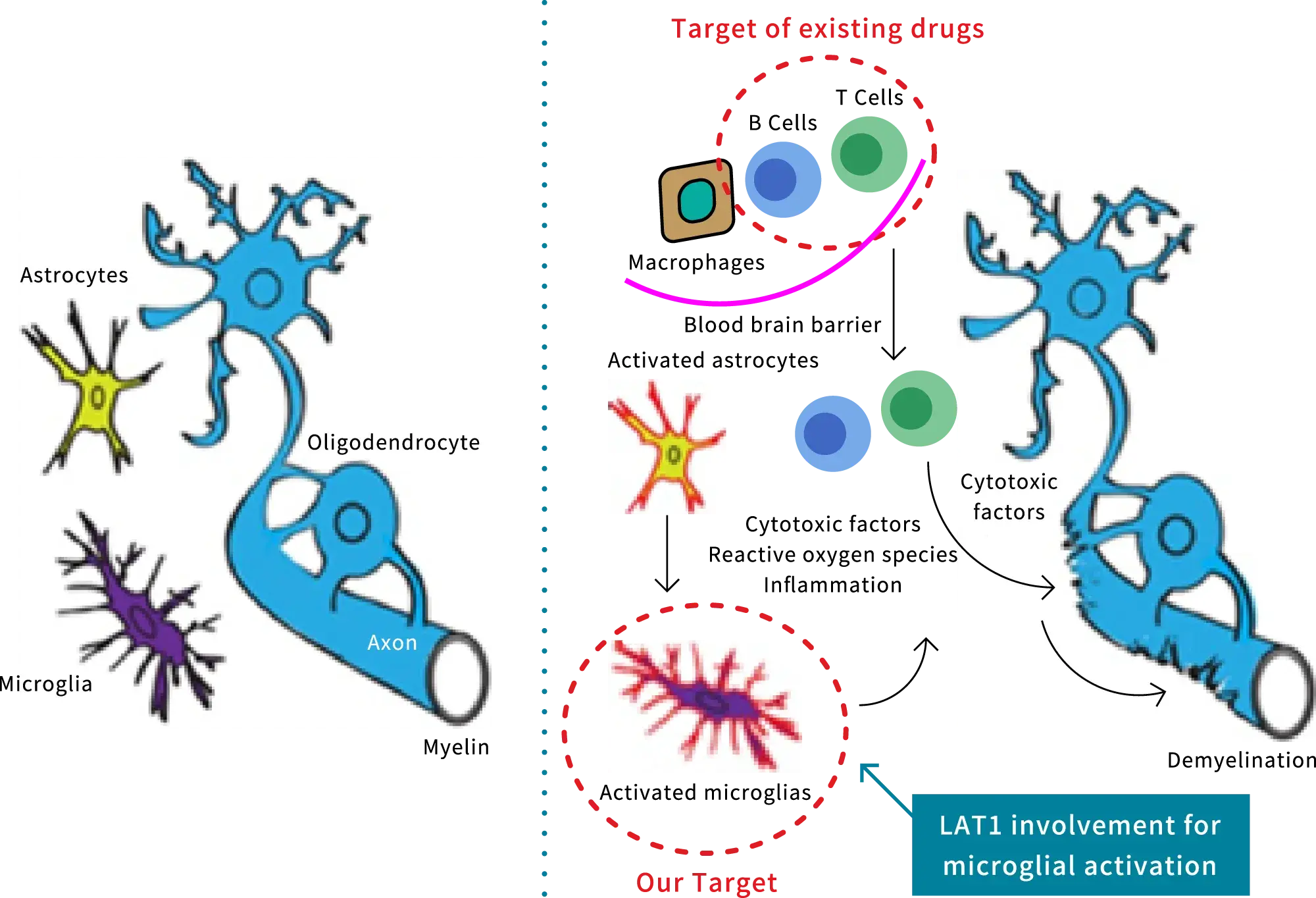
JPH034, an allosteric LAT1 inhibitor, was discovered by Professor Yoshikatsu Kanai and his colleagues at the Osaka University Graduate School of Medicine. J-Pharma has been granted an exclusive ordinary license and is developing it for the treatment of progressive multiple sclerosis (MS). Leveraging its characteristics of excellent brain migration and suppression of microglia activity, JPH034 has the potential to reduce chronic inflammation, which is not targeted by marketed drugs, and thus improve symptoms and control disease progression.
J-Pharma has received a grant from the National Multiple Sclerosis Society’s Fast Forward Commercial Funding Program in 2023 to support the development of JPH034. JPH034 has been developed in collaboration with several universities in the U.S. and Europe, which have published various research results in the MS field around the world. Collaborative research with a U.S. university demonstrated that JPH034 suppressed local inflammation in the demyelinating lesion area of the central nervous system (CNS) and improved clinical scores along with remyelination, suggesting its efficacy in multiple sclerosis. For further exploration, a clinical study without drug administration is underway at a European university to determine whether microglial activity, an inflammatory factor in the CNS, and LAT1 expression coexist at the demyelinating lesion level. This study aims to identify a patient population in which JPH034 can both inhibit the progression of progressive MS (by reducing inflammation and promoting remyelination) and improve function (by enhancing EDSS scores) as its therapeutic effect. Preparations for the Ph.1 study are also underway.
Multiple sclerosis (MS) and JPH034
MS is a chronic disease of the CNS that typically develops in young adult females, and more than 100,000 people worldwide are diagnosed each year. Progressive MS causes sensory disturbance, visual impairements, and motor paralysis, which may result in limb disability and wheelchair-bound daily living. It is designated as an intractable disease by the Ministry of Health, Labour, and Welfare of Japan. MS is categorized into three types: the relapsing-remitting type (RRMS), the secondary progressive type (SPMS), and the primary progressive type (PPMS). While there are numerous marketed drugs primarily targeting RRMS, there are currently no effective treatments for SPMS or PPMS. Notably, approximately half of untreated RRMS cases progress to SPMS.
It is generally recognized that activation of B cells and T cells, as well as the activation of microglia, are factors that aggravate the disease state of MS. Marketed drugs target B cells and T cells in relapsing-remitting MS that migrate from the periphery to the brain, effectively suppressing active inflammation caused by their activation. However, these drugs are unable to treat chronic local inflammation in secondary progressive MS that progresses due to the activation of microglia resident in the brain. This leads to the progressive deterioration of the disease resulting in irreversible neurological and motor dysfunction. JPH034 targets microglia activation, which is not addressed by marketed drugs. As a result, it reduces chronic local inflammation and enhances remyelination, leading to symptom improvement and a slower progression of the disease.
Basic mechanism of MS development and drug targets