2025.10.19
2025 European Society for Medical Oncology (ESMO) Annual Congress: Presentation of Subgroup Analysis Results from the Phase 2 Clinical Trial of Nanvuranlat Suggesting Patient Selection Criteria for the Global Phase 3 Clinical Trial
October 19, 2025
J-Pharma Co., Ltd.
J-Pharma Co., Ltd. announces that the results of subgroup analysis and exposure–response (ER) analysis from the Phase 2 clinical study of nanvuranlat, an L-type amino acid transporter 1 (LAT1) inhibitor for advanced biliary tract cancer, were presented at the 2025 European Society for Medical Oncology (ESMO 2025) Annual Congress held in Berlin, Germany, from October 17 to 21.
These analyses suggest criteria for selecting appropriate patients for treatment with nanvuranlat. By appropriately reflecting these findings in the trial design, J-Pharma expects to enhance the probability of success for the planned global Phase 3 clinical trial, scheduled to commence within this fiscal year.
Poster session
Date & Time: October 19, 2025, 12:00–12:45 (local time)
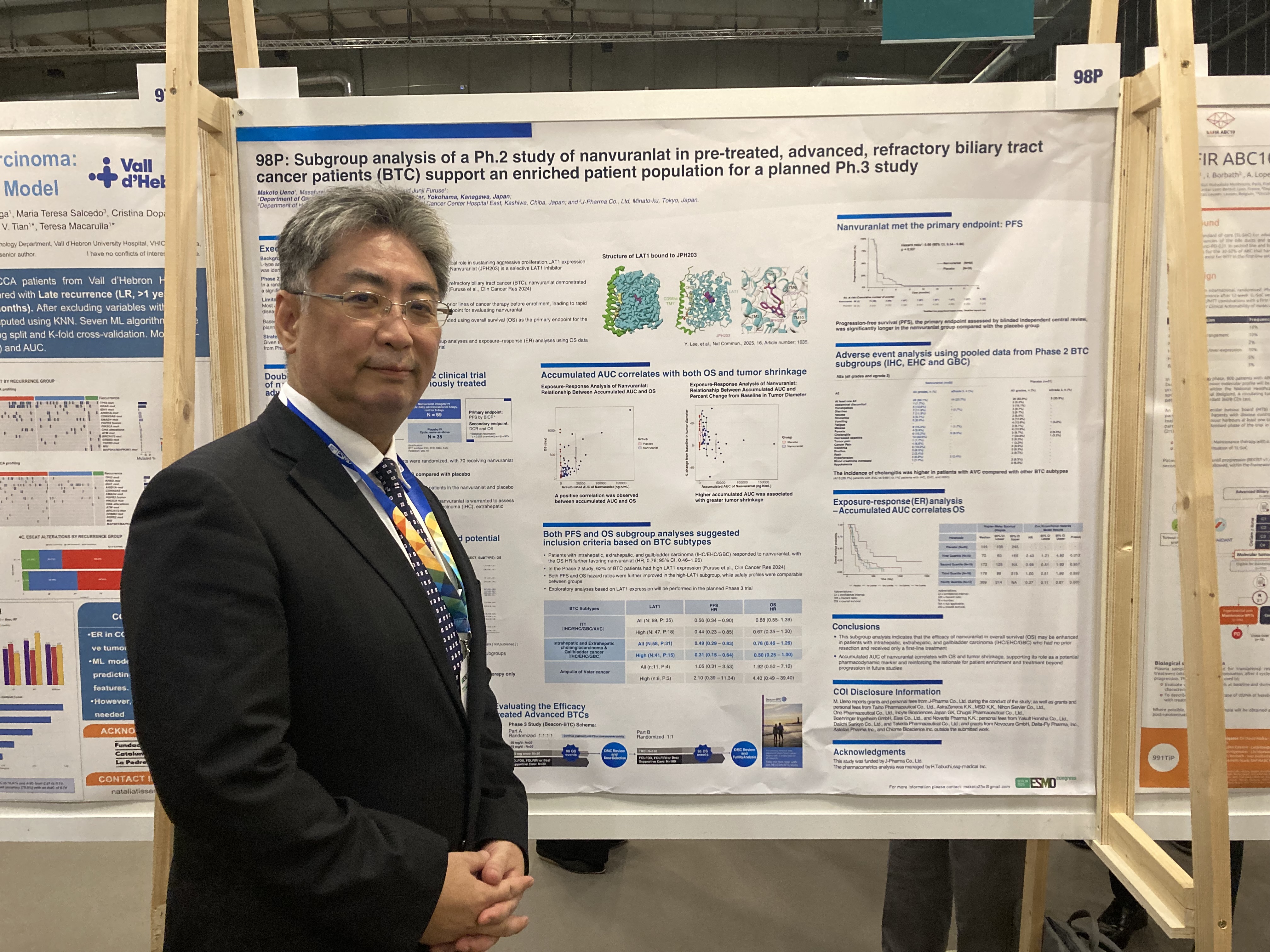
Title: Subgroup analysis of a Ph. 2 study of Nanvuranlat in pre-treated, advanced, refractory biliary tract cancer patients (BTC) support an enriched patient population for a planned Ph. 3 study
Presenter: Dr. Makoto Ueno, Director, Department of Gastroenterology, Kanagawa Cancer Center
Main Presentation Content:
・In intrahepatic cholangiocarcinoma (IHC), extrahepatic cholangiocarcinoma (EHC), and gallbladder cancer (GBC), the nanvuranlat group showed a favorable trend toward prolonged overall survival (OS) compared to the placebo group (hazard ratio = 0.76; 95% confidence interval: 0.46–1.26).
・When limiting the analysis to patients receiving second-line therapy (excluding those receiving third-line or later therapy), the nanvuranlat group showed a favorable trend toward OS prolongation compared to the placebo group (hazard ratio = 0.55; 95% confidence interval: 0.09–3.54).
・Among patients without prior resection of the primary tumor, the nanvuranlat group showed a favorable trend toward OS prolongation compared to the placebo group (hazard ratio = 0.53; 95% confidence interval: 0.228–1.01).
・In the nanvuranlat group, patients with higher accumulated AUC (drug exposure) showed greater tumor shrinkage effects and a tendency toward prolonged overall survival (OS).
(Reference)
About Nanvuranlat
Nanvuranlat is a novel LAT1-specific inhibitor originally discovered by J-Pharma and is the first small-molecule compound of its kind to be clinically developed worldwide. If approved as a pharmaceutical product, it has the potential to become a first-in-class drug with a novel mechanism of action for its target disease.
Since 2015, J-Pharma has conducted Phase 1 clinical studies in multiple solid tumors and identified its potential for the treatment of advanced biliary tract cancer. A Phase 2 clinical study targeting advanced biliary tract cancer was conducted starting in 2018, demonstrating that nanvuranlat monotherapy provides clinically meaningful benefit. *
Nanvuranlat was designated as an orphan drug by the U.S. Food and Drug Administration (FDA) in April 2022. Furthermore, on September 25, 2024, the FDA approved the Investigational New Drug (IND) application for clinical studies in cancer patients. In addition, in May 2025, J-Pharma confirmed that the Chemistry, Manufacturing, and Controls (CMC) at the commercial manufacturing scale meet the quality standards required by the FDA. Preparations are now underway for the initiation of the global Phase 3 clinical study scheduled to commence in this fiscal year (2025).
* Publication on the results of the Japan Phase II clinical study of Nanvuranlat:
Furuse et al. A Phase II Placebo-Controlled Study of the Effect and Safety of Nanvuranlat in Patients with Advanced Biliary Tract Cancers Previously Treated by Systemic Chemotherapy. Clin Cancer Res. 2024; 30(18):3990–3995.
About J-Pharma Co., Ltd.
J-Pharma Co., Ltd. aims to pursue new possibilities for SLC transporters and contribute to the hope and health of people worldwide through the development of innovative new drugs that address unmet medical needs. Under this mission, the Company has focused on LAT1 (L-type amino acid transporter 1), one of the SLC transporters discovered by the Company’s founder and is advancing the development of LAT1 inhibitors to address the needs of patients with cancer and autoimmune diseases, where existing drugs are insufficient.
For more information, please visit: https://www.j-pharma.com/en/
Inquiries:
J-Pharma Co., Ltd.
Planning Department
TEL : +81-3-6432-4270
https://www.j-pharma.com/en/contact